In a collaboration with Prof. Beck (Head of Neurosurgery Freiburg), we recently demonstrated plasticity of excitatory neurotransmission in the human neocortex (Lenz et al., Elife 2021), and found that synaptic plasticity is accompanied by ultrastructural changes of dendritic spines and a peculiar intracellular organelle found in a subset of spines – the spine apparatus organelle (SA).
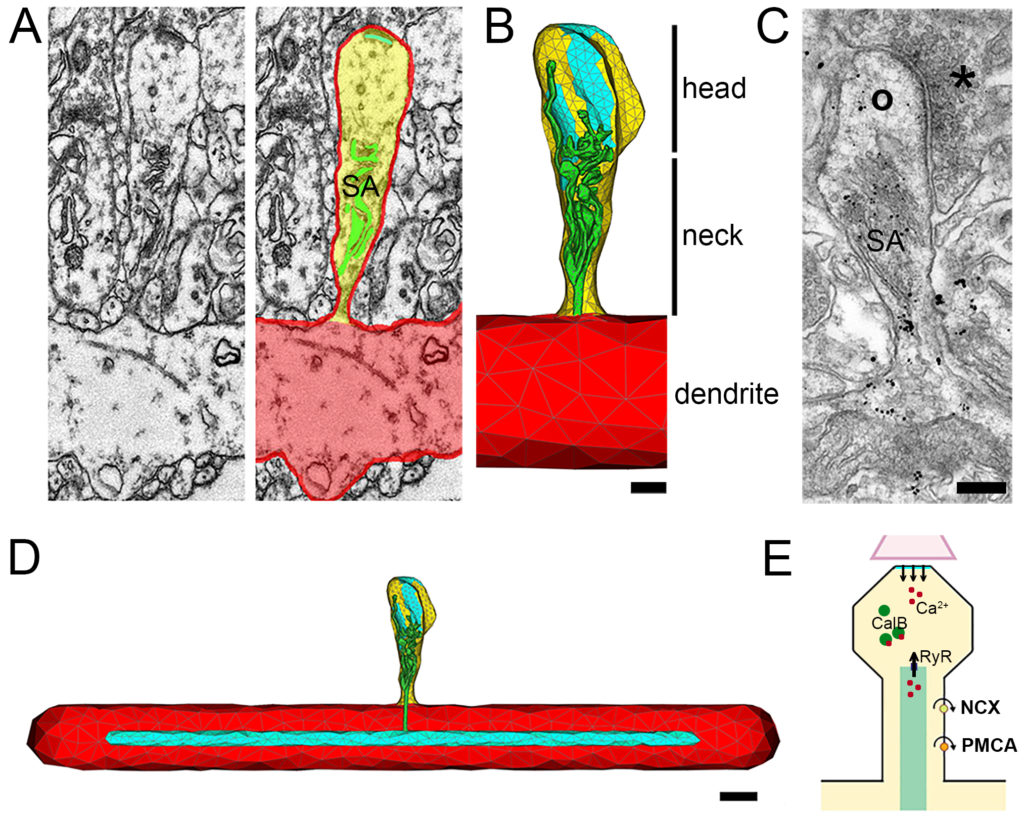
Using serial transmission electron microscopy, 3D reconstructions and computer simulations of human dendritic spines in collaboration with the Prof. Haas, Prof. Beck (both Neurosurgery, Freiburg), and Prof. Queisser (Mathematics, Temple-University, USA), Viet Duc Bui (cand. med.; Neuroanatomy, Freiburg) and James Rosado (PhD candidate; Mathematics, Temple University, USA) studied the impact of human SAs on spine-to-dendrite calcium signaling, which plays a fundamental role in synaptic plasticity.
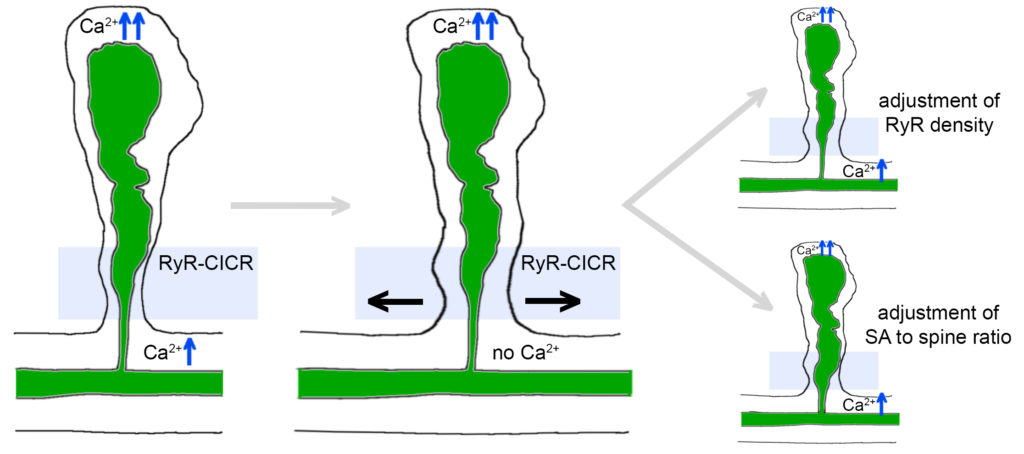
Our simulations revealed an “all-or-nothing” Ca2+ communication switch between spine heads and dendrites that is controlled by the interplay between Ryanodine Receptor densities, and the morphology of dendritic spines and SA in the spine neck region. These findings suggest that structural changes of SAs can serve calcium homeostasis during dendritic spine growth and may act as a structural switch for input-specific synaptic plasticity in human dendritic spines.
Rosado J*, Duc Bui V*, Haas CA, Beck J, Queisser G, Vlachos A (2022) Calcium modeling of spine apparatus-containing human dendritic spines demonstrates an “all-or-nothing” communication switch between the spine head and dendrite. PLoS Comput Biol 18(4): e1010069. https://doi.org/10.1371/journal.pcbi.1010069 *joint first authors
Link to the original publication: https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1010069
